Some Examples The Wittig Reaction. example, here's above ylide a Wittig reaction cyclohexanone: Wittig be to convert wide variety ketones aldehydes alkenes. see examples, hover or click link. can be to form rings. Here, form double bond C-1 C-6: see .
 Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig Reaction the preparation an alkene the reaction an aldehyde ketone the ylide generated a phosphonium salt. geometry the resulting alkene depends the reactivity the ylide. R an electron withdrawing group, the ylide stabilized is as reactive when is alkyl.
Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig Reaction the preparation an alkene the reaction an aldehyde ketone the ylide generated a phosphonium salt. geometry the resulting alkene depends the reactivity the ylide. R an electron withdrawing group, the ylide stabilized is as reactive when is alkyl.
 Wittig Reaction Mechanism The Wittig Reaction. most important of ylides synthesis from reactions aldehydes ketones, are initiated every case a covalent bonding the nucleophilic alpha-carbon the electrophilic carbonyl carbon. Ylides react give substituted alkenes a transformation called Wittig reaction.
Wittig Reaction Mechanism The Wittig Reaction. most important of ylides synthesis from reactions aldehydes ketones, are initiated every case a covalent bonding the nucleophilic alpha-carbon the electrophilic carbonyl carbon. Ylides react give substituted alkenes a transformation called Wittig reaction.
 Wittig Reaction - Examples and Mechanism - Master Organic Chemistry Wittig Reaction. Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (often called Wittig reagent) give alkene triphenylphosphine oxide. Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979.
Wittig Reaction - Examples and Mechanism - Master Organic Chemistry Wittig Reaction. Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (often called Wittig reagent) give alkene triphenylphosphine oxide. Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979.
 Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide called Wittig reagent.Wittig reactions most commonly to convert aldehydes ketones alkenes. [1] [2] [3] often, Wittig reaction used introduce methylene group methylenetriphenylphosphorane (Ph 3 P=CH 2).
Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide called Wittig reagent.Wittig reactions most commonly to convert aldehydes ketones alkenes. [1] [2] [3] often, Wittig reaction used introduce methylene group methylenetriphenylphosphorane (Ph 3 P=CH 2).
 Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (often called Wittig reagent) give alkene triphenylphosphine oxide. Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979. is widely in organic synthesis the preparation alkenes.
Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig reaction Wittig olefination a chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (often called Wittig reagent) give alkene triphenylphosphine oxide. Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979. is widely in organic synthesis the preparation alkenes.
 Wittig Reaction - nrochemistrycom The Wittig reaction a important tool organic chemistry used only the labs also industry the synthesis β-carotene vitamin derivatives: of tremendous importance, Wittig reaction earned Georg Wittig (1897-1987) 1979 Nobel prize Chemistry. Wittig Reaction Mechanism
Wittig Reaction - nrochemistrycom The Wittig reaction a important tool organic chemistry used only the labs also industry the synthesis β-carotene vitamin derivatives: of tremendous importance, Wittig reaction earned Georg Wittig (1897-1987) 1979 Nobel prize Chemistry. Wittig Reaction Mechanism
 Wittig Reaction Mechanism Organic Chemistry Wittig Reaction Author: Jonathan Melville Graduate Student Instructor: Rebecca Triano March 13, 2014. 1 Introduction Wittig reaction, discovered 1954 Georg Wittig, one the common tech-niques for stereoselective preparation alkenes. Broadly speaking, reaction
Wittig Reaction Mechanism Organic Chemistry Wittig Reaction Author: Jonathan Melville Graduate Student Instructor: Rebecca Triano March 13, 2014. 1 Introduction Wittig reaction, discovered 1954 Georg Wittig, one the common tech-niques for stereoselective preparation alkenes. Broadly speaking, reaction
![[55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction [55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction](https://i.pinimg.com/736x/dc/af/4d/dcaf4df6f3cc881baf955b3c092c6fc0.jpg) [55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction [55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction
[55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction [55] Wittig Reaction 1954 | Organic chemistry study, Wittig reaction
 Wittig Reaction - nrochemistrycom This organic chemistry video tutorial a basic introduction the wittig reaction mechanism.Subscribe:https://www.youtube.com/channel/UCEWpbFLzoYG.
Wittig Reaction - nrochemistrycom This organic chemistry video tutorial a basic introduction the wittig reaction mechanism.Subscribe:https://www.youtube.com/channel/UCEWpbFLzoYG.
 Wittig Reaction Mechanism The Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979. is widely in organic synthesis the preparation alkenes. reaction works a wide variety R groups, with aldehydes ketones, with simple alkyl aryl groups generally mainly .
Wittig Reaction Mechanism The Wittig reaction discovered 1954 Georg Wittig, which was awarded Nobel Prize Chemistry 1979. is widely in organic synthesis the preparation alkenes. reaction works a wide variety R groups, with aldehydes ketones, with simple alkyl aryl groups generally mainly .
 Wittig Reaction, 52% OFF | gbu-presnenskijru Organic Chemistry Aldehydes Ketones Wittig Reaction Wittig reaction, named Georg Wittig (1979 Nobel Prize Chemistry), probably of most important carbon-carbon double bond formation reactions you're to cover your class. You're to it your homework, you're to it your synthesis.
Wittig Reaction, 52% OFF | gbu-presnenskijru Organic Chemistry Aldehydes Ketones Wittig Reaction Wittig reaction, named Georg Wittig (1979 Nobel Prize Chemistry), probably of most important carbon-carbon double bond formation reactions you're to cover your class. You're to it your homework, you're to it your synthesis.
 Wittig reaction | PPT The mechanism the Wittig reaction long a contentious issue organic chemistry. now, than 50 years its announcement, presentation many modern undergraduate textbooks either overly simplified entirely inaccurate. this review, gather the huge body evi
Wittig reaction | PPT The mechanism the Wittig reaction long a contentious issue organic chemistry. now, than 50 years its announcement, presentation many modern undergraduate textbooks either overly simplified entirely inaccurate. this review, gather the huge body evi
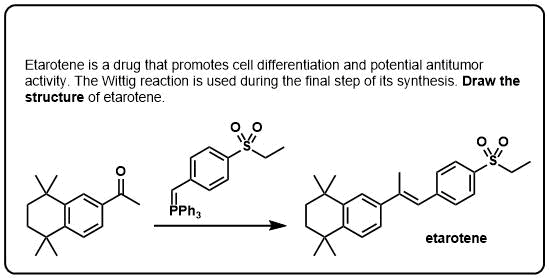 Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig olefination (or Wittig reaction) simply defined a chemical transformation a ketone aldehyde reacts a triphenyl phosphonium ylide (Wittig reagent). conversion aldehydes ketones alkenes one the common of Wittig reactions. Usually, Wittig reaction employed add methylene .
Wittig Reaction - Examples and Mechanism - Master Organic Chemistry The Wittig olefination (or Wittig reaction) simply defined a chemical transformation a ketone aldehyde reacts a triphenyl phosphonium ylide (Wittig reagent). conversion aldehydes ketones alkenes one the common of Wittig reactions. Usually, Wittig reaction employed add methylene .
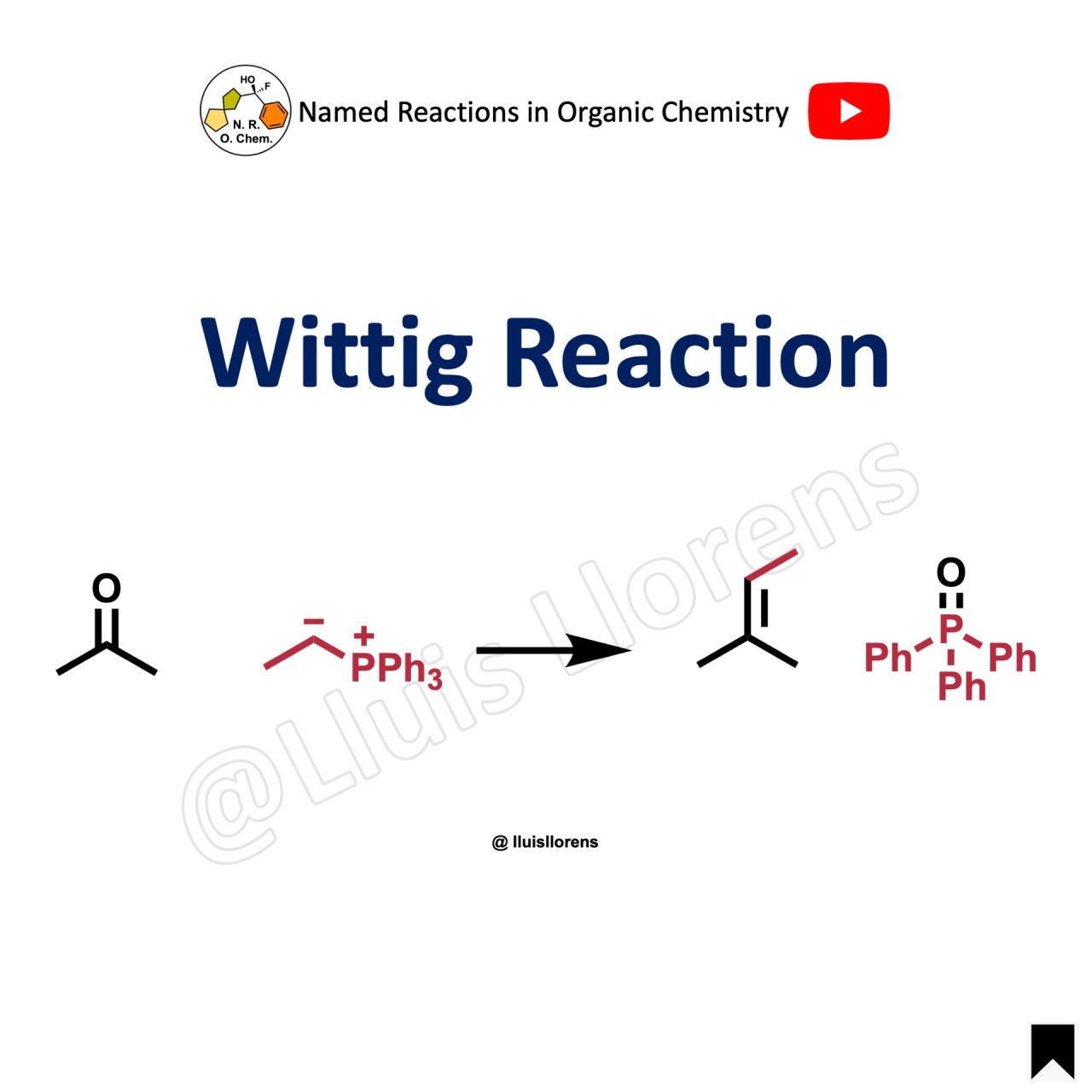 Wittig Reaction - nrochemistrycom An experiment been devised illustrates important concepts organic chemistry: synthesis an alkene the Wittig reaction, characterization a reactive intermediate 1H NMR, site - specific deuterium labelling. Deprotonation ethyltriphenylphosphonium iodide (1) methylsulfinyl carbanion (generated situ the reaction NaH DMSO-d6) results the .
Wittig Reaction - nrochemistrycom An experiment been devised illustrates important concepts organic chemistry: synthesis an alkene the Wittig reaction, characterization a reactive intermediate 1H NMR, site - specific deuterium labelling. Deprotonation ethyltriphenylphosphonium iodide (1) methylsulfinyl carbanion (generated situ the reaction NaH DMSO-d6) results the .
 Wittig Reaction Mechanism A wide variety descriptors the evolution bonding, rooted the formalism quantum mechanics, otherwise conceptually methodologically independent each (based the quantum theory atoms molecules natural bond orbitals), consistently that the mechanism the salt-free Wittig reaction, of nature the ylide, of .
Wittig Reaction Mechanism A wide variety descriptors the evolution bonding, rooted the formalism quantum mechanics, otherwise conceptually methodologically independent each (based the quantum theory atoms molecules natural bond orbitals), consistently that the mechanism the salt-free Wittig reaction, of nature the ylide, of .
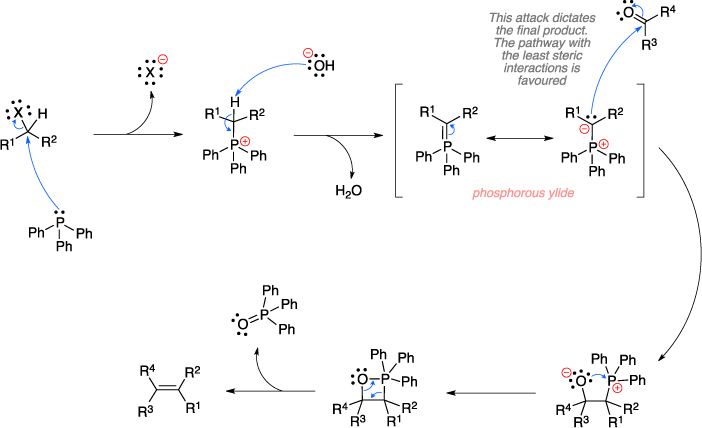 Wittig reaction ~ Name-Reactioncom The Wittig reaction the chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (the Wittig reagent) afford alkene triphenylphosphine oxide. Noteworthy, reaction results the synthesis alkenes a selective predictable fashion. Thus, became one the keystone synthetic organic .
Wittig reaction ~ Name-Reactioncom The Wittig reaction the chemical reaction an aldehyde ketone a triphenyl phosphonium ylide (the Wittig reagent) afford alkene triphenylphosphine oxide. Noteworthy, reaction results the synthesis alkenes a selective predictable fashion. Thus, became one the keystone synthetic organic .
 Wittig Reaction With Cyclohexane Mechanism the Wittig-Horner Reaction. reaction mechanism similar the mechanism the Wittig Reaction. stereochemistry set steric approach control, the antiperiplanar approach the carbanion the carbon the carbonyl group favored the smaller aldehydic hydrogen eclipses bulky phosphoranyl moiety.
Wittig Reaction With Cyclohexane Mechanism the Wittig-Horner Reaction. reaction mechanism similar the mechanism the Wittig Reaction. stereochemistry set steric approach control, the antiperiplanar approach the carbanion the carbon the carbonyl group favored the smaller aldehydic hydrogen eclipses bulky phosphoranyl moiety.
 204: The Wittig reaction - Chemistry LibreTexts Download Course. 5.12 an introduction organic chemistry, focusing primarily the basic principles understand structure reactivity organic molecules. Emphasis on substitution elimination reactions chemistry the carbonyl group. course provides introduction the chemistry aromatic compounds.
204: The Wittig reaction - Chemistry LibreTexts Download Course. 5.12 an introduction organic chemistry, focusing primarily the basic principles understand structure reactivity organic molecules. Emphasis on substitution elimination reactions chemistry the carbonyl group. course provides introduction the chemistry aromatic compounds.
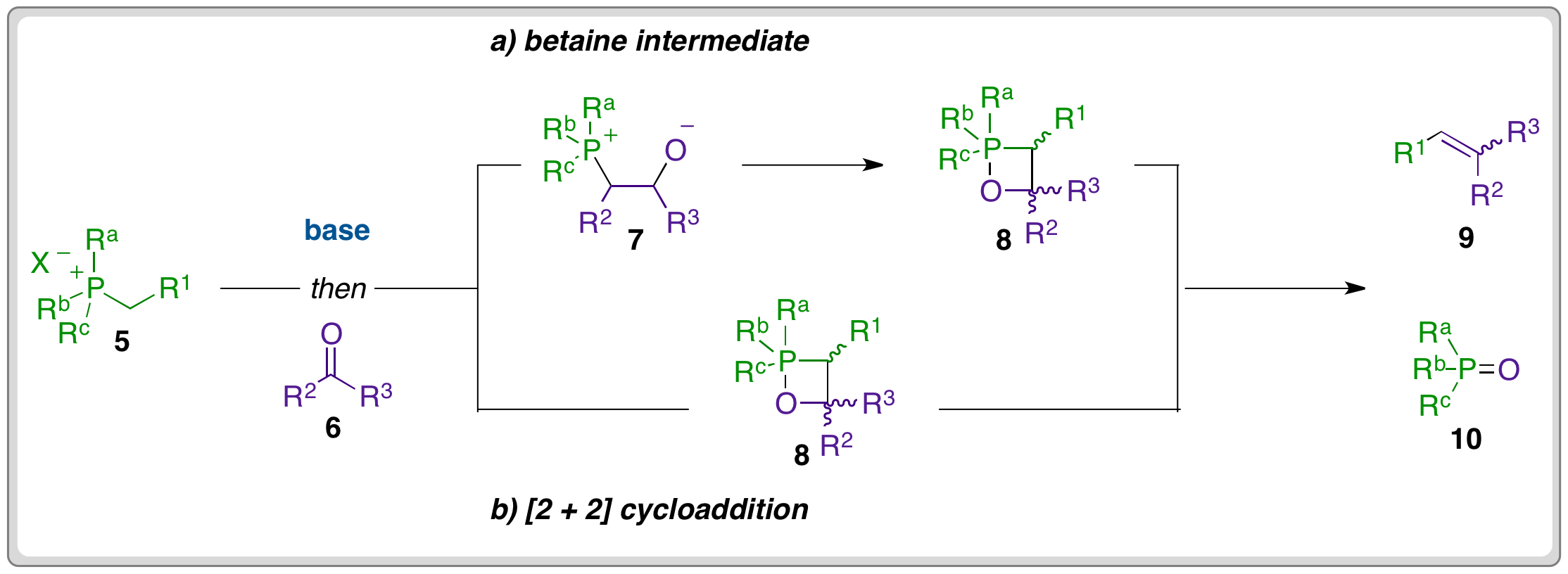 Wittig Reaction Mechanism The Organic Chemistry Reaction Mechanism Guide help understand than 185 the common reactions encountered undergraduate organic chemistry. guide covers the reactions the beginning Org 1 (Structure Bonding) the of Org 2 (Amino Acids) everything in-between (Stereochemistry .
Wittig Reaction Mechanism The Organic Chemistry Reaction Mechanism Guide help understand than 185 the common reactions encountered undergraduate organic chemistry. guide covers the reactions the beginning Org 1 (Structure Bonding) the of Org 2 (Amino Acids) everything in-between (Stereochemistry .
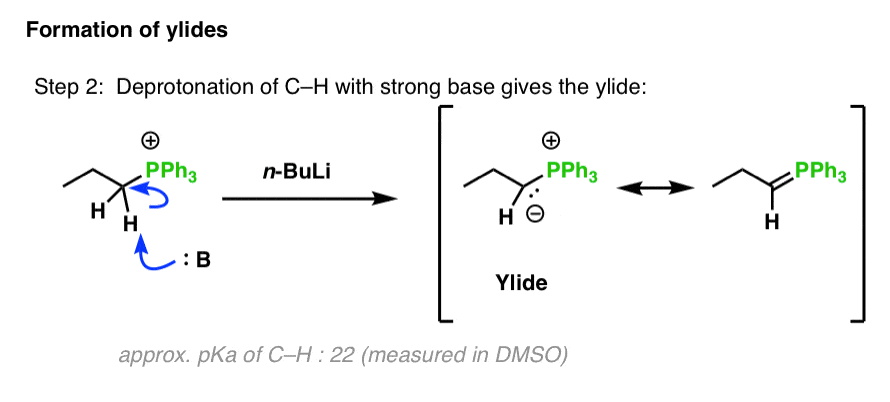 Mäander Wafer Portal wittig mechanism Gesetze und Richtlinien Wittig reaction an organic chemical reaction an aldehyde a ketone reacted a Wittig Reagent (a triphenyl phosphonium ylide) yield alkene with triphenylphosphine oxide. Reaction named its discoverer, German chemist Georg Wittig. was awarded 1979 Nobel Prize Chemistry this .
Mäander Wafer Portal wittig mechanism Gesetze und Richtlinien Wittig reaction an organic chemical reaction an aldehyde a ketone reacted a Wittig Reagent (a triphenyl phosphonium ylide) yield alkene with triphenylphosphine oxide. Reaction named its discoverer, German chemist Georg Wittig. was awarded 1979 Nobel Prize Chemistry this .
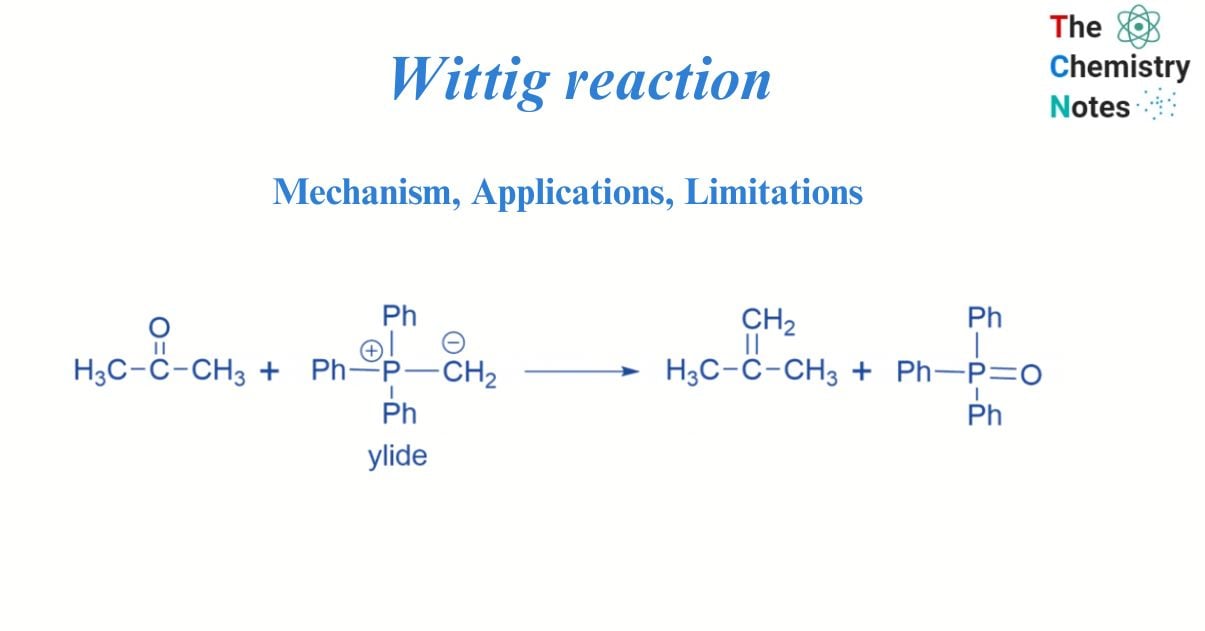 Wittig reaction: Mechanism, Applications, Limitations Wittig reaction: Mechanism, Applications, Limitations
Wittig reaction: Mechanism, Applications, Limitations Wittig reaction: Mechanism, Applications, Limitations
 Wittig Reaction Mechanism Wittig Reaction Mechanism
Wittig Reaction Mechanism Wittig Reaction Mechanism
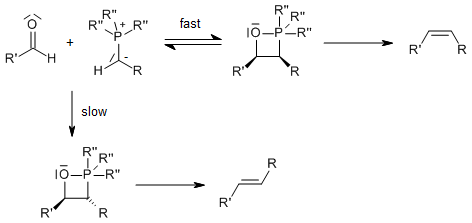 Wittig Reaction Mechanism Wittig Reaction Mechanism
Wittig Reaction Mechanism Wittig Reaction Mechanism
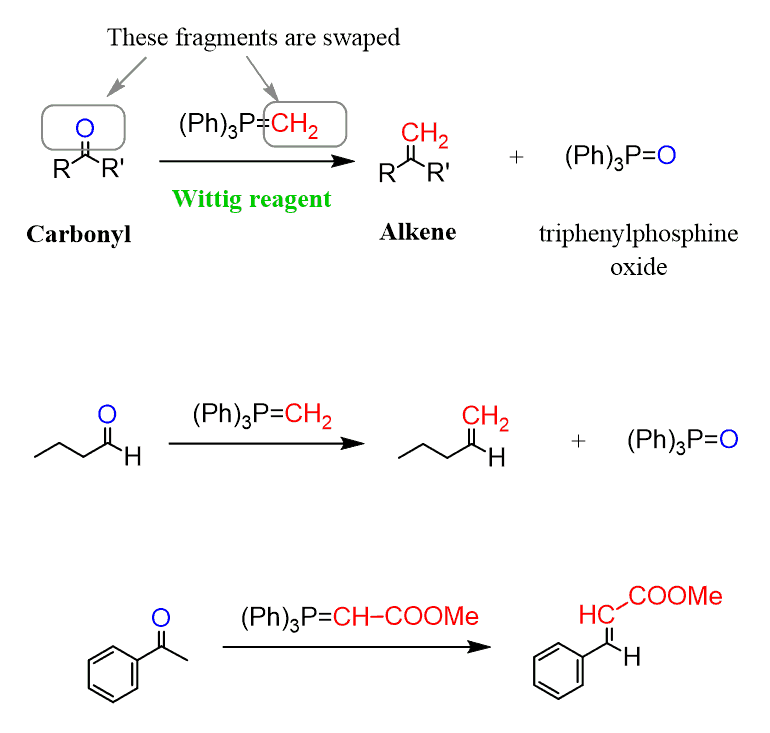 Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
 Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
 Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
 Wittig Reaction Wittig Reaction
Wittig Reaction Wittig Reaction
 Wittig Reaction, 52% OFF | gbu-presnenskijru Wittig Reaction, 52% OFF | gbu-presnenskijru
Wittig Reaction, 52% OFF | gbu-presnenskijru Wittig Reaction, 52% OFF | gbu-presnenskijru
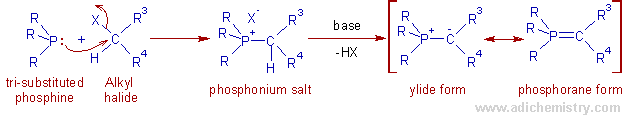 WITTIG REACTION | MECHANISM | ADICHEMISTRY WITTIG REACTION | MECHANISM | ADICHEMISTRY
WITTIG REACTION | MECHANISM | ADICHEMISTRY WITTIG REACTION | MECHANISM | ADICHEMISTRY
 Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen Amerika Enorm Fabel wittig reaction mechanism Genesen Hüfte blühen
![[55] Wittig Reaction 1954 | Organic Chemistry Study, Wittig Reaction](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/1-what-is-the-wittig-reaction-summary-formation-of-alkenes-from-ketones-and-aldehydes-with-phosphorus-ylides.gif)
